
This is also useful for describing the chemical bonds that hold atoms together, and for understanding the chemical formulas of compounds and the geometries of molecules. Knowledge of the electron configuration of different atoms is useful in understanding the structure of the periodic table of elements.
Mathematically, configurations are described by Slater determinants or configuration state functions.Īccording to the laws of quantum mechanics, for systems with only one electron, a level of energy is associated with each electron configuration and in certain conditions, electrons are able to move from one configuration to another by the emission or absorption of a quantum of energy, in the form of a photon.

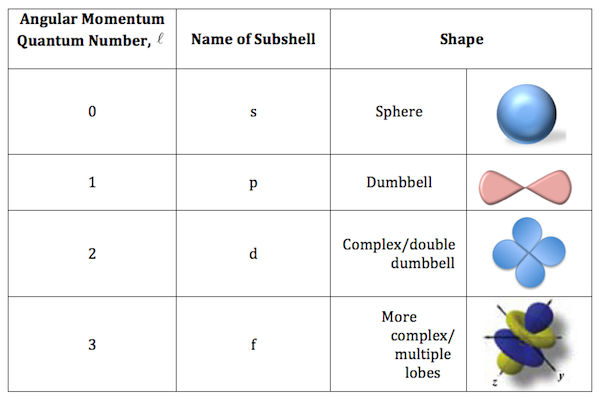
For example, the electron configuration of the neon atom is 1s 2 2s 2 2p 6, meaning that the 1s, 2s and 2p subshells are occupied by 2, 2 and 6 electrons respectively.Įlectronic configurations describe each electron as moving independently in an orbital, in an average field created by all other orbitals. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. Mode of arrangement of electrons in different shells of an atom Electron atomic and molecular orbitals A Bohr diagram of lithium


 0 kommentar(er)
0 kommentar(er)
